The Universal Solvent
22 Oct
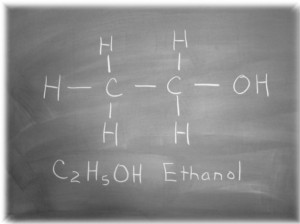
Ethanol is part of our every day lives. It’s mixed in with gasoline, used for cleaning, and of course, consumed in copious amounts. For those of us in the bar world, we know it a bit more intimately than others. As we delve ever deeper into the world of flavor and the science there of, let’s take a moment to recognize the amazing properties of this little cluster of Carbon, Hydrogen, and Oxygen.
The Universal Solvent
Ethanol is an incredible substance. We’re aware of it’s physical effects on us, but what about it as a chemical? Alcohol is a very good solvent for a variety of things. It’s extremely good at dissolving esters, sugars, light hydrocarbons like pentane, and acids. It’s also hygroscopic, which is a really fancy way of saying Ethanol and water love each other very much. It’s almost impossible to isolate alcohol from water entirely, and even if one does, it will wick water out of the air until it becomes about 2-3% water by volume. This explains why we can only buy 195 proof everclear and not more.
Because alcohol and water are almost always found together, that means that the solubility properties of each is compounded with the other. For example, alcohol can dissolve oils but water can not. In a high alcohol to water mixture, a significant amount of oil can be dissolved. For a good example, just imagine adding water to absinthe drop by drop and watching the mixture get cloudy. This effect is caused by the oils precipitating out of solution as the proof of the mixture drops.
Ok, Mr. Science guy, that’s all fine and good. But what does any of this mean practically? Well it just so happens that all this stuff that alcohol is so good at dissolving has flavor. This makes spirits a perfect platform to capture the essence of the distilled product, aging, adjuncts or added aromatics. Ethanol’s ability to dissolve oils combined with water’s ability to dissolve sugars allows spirits to have very rich viscosity which leads to a creamy texture. Congeners and esters left by the distilling process impart the spirit with flavor and aroma.
Simply put, Ethanol allows booze to taste delicious.Which is why I love booze so very, very much. This brings us to my least favorite category of distilled spirit.
I’ve always known that vodka isn’t my choice of spirit. Unlike most, I never went through a phase in which vodka was my tipple of choice. I went from spiced rum to gin to beer to whiskey to tequila, and finally arriving at a healthy appreciation for almost all spirits categories. All save one.
A Spiritual Outlier
The issue that I’ve always had with vodka is simple. I first verbalized it in the caricature that vodka doesn’t taste like anything. But as I’ve learned more about how flavor works, I’ve come to realize that that simple dismissal needs a bit more examination.
Let’s first focus on the process of making modern vodka. By any assessment it is a heavily rectified spirit. Distilled anywhere from a reasonable to a comical number of times, the goal of the process of vodka is to eliminate as many impurities as possible, creating a product neutral in flavor with low perceived heat. The same process by which impurities are removed affect both the flavor and the texture of the resulting spirit. Neutrality is achieved usually at the cost of any residual oils, sugars, or acids which would give the spirit texture. It is for this reason that I’ve always felt vodka to have an unsatisfying mouth feel.
As for vodka and it’s purported neutrality, I’ve never fully bought the idea that it’s flavor can be masked in a drink or by a mixer. Vodka with coke surely tastes different than just coke. So what, then, is vodka bringing to the table flavor-wise? The centerpiece of vodka’s flavor profile is the alcohol itself. Which leads us back to where we began: alcohol as a solvent.
In spirits, wine, or beer tasting vernacular, solvent is always a fault. If someone calls out solvent and someone else confirms that, the investigation then begins as to what went wrong with this beverage. In spirits, the presence of acetone or methanol can indicate a problem in the heads of the distillate. In wine, Ethyl acetate can be produced by microbes, giving the wine a varnish aroma.
Ethanol, stripped of all the things it’s good at dissolving, has a distinct subtle solvent aroma. I find this flavor much more present in drinks made from a highly rectified spirit versus those made with spirits not distilled to neutrality.
A Hope from the Past
Vodka as a category has strayed a long way from where it started. Before the advent of the coffee still in 1822, it was impossible to distill vodka to complete neutrality. Pot stills are beautifully primitive devices that after a point make it increasingly difficult to raise the proof of the resulting spirit. Also, distilling each additional time in a pot still is a major undertaking, versus the ease of passing a spirit once again through a column still.
This means that before the mid-1800s, vodka was very much a spirit that contained the essence of materials from which it was made. It was richer, fattier, and more flavorful than the products available to us today. In fact, vodka at that time had far more in common with white whiskey than it does with vodka’s current iteration.
Our trip to Chopin distillery showed me that there are people out there looking towards the future of the vodka category. Chopin is soon to release a line of single distilled vodkas, the very intent of which is flavor and character. Each product uses different raw materials, and the resultant spirit reflects the beautiful aromatics of the source, be it potato or grain.
While I wish I could forecast the beginning of a vodka Renascence, my hopes are tempered with the dominance of highly rectified vodka in the market. Regardless, I rest assured that cool products are in the pipeline that can find a place on my back bar and win over this bartender’s affections to his formerly-least favorite category.

One Response to “The Universal Solvent”